Do the receptacle 99R10-01A1 made out of zinc has galvanic corrosion with cress 300 or other stainless steel components
- English
- Français
- Nederlands
- Español
- Deutsch
- Português
Question:
I am looking for hardware to help me make an assembly.
I am designing a little door that is made from Fiber glass that is 0.25in thick where it will go the rivetless receptacle. I was considering the 99R10-01A1 that is made of Zinc. The door will be made of CRES 304, 0.060in thick, and on this the 1/4 turn stud will be located to close the door.
Doo you think that the receptacle make out of Zinc and the other components make out of CRES have a galvanic corrosion?
Answer:
Thank you for your concerns about galvanic corrosion.
Indeed this can be an issue for many applications however, see in the link below more in depth information:
When you read this, these are the conclusions:
- When 300 series are in contact with zinc, neither material will suffer additional corrosion, or at the most only slight corrosion which is tolerable
- Since the stud is made of stainless steel: you will have figure 2. The fastener should not fail due to corrosion.
So I can advise that these will go together.
Important note: If the two plates that you wish to connect are not in contact with each other, and only through the fastener then, you might have more chances of corrosion.
I have however only seen on the outside of trains where there is a lot of wind passing causing static electricity that makes the parts corrode.
Here is the complete article:
Stainless steel and galvanized materials often are found together in industry with applications such as galvanized fasteners, stainless steel pressure vessels and roof and siding panels. Do I need to worry about these two metals corroding each other? What other concerns should I have pertaining to hot dip galvannized steel and stainless steel in contact?
Does glavanic corrosion occur when these metals are in contact?
The effect of having zinc and stainles steel in contact while being exposed to the same electrolyte causes the oxidation reaction to occur primarily at the zinc surface and the reduction reaction at the stainless steel surface. As a result, corrosion of the more noble metal (stainless steel) is decreased and that of the more active meatl (zinc) increased.
However, when austenitic stainless steels (300 series) are in contact with zinc, neither material will suffer additional corrosion, or at the most, only slight corrosion. The slight corrosion is usually tolerarable when in this bimetallic contact. There is one exception to this conclusion as follows.
Any time a bimetallic assembly contains metal systems that are subject to galvanic corrosion, as we have here, the ration of the cathodic area (stainless steel) to that of the anode (zinc) must be carefully considered. The corrosion current that flows between the cathode and anode is independent of area, but the rate of penetrationat the anotde is dependant on the current per unit area, that is current density. Therfore, it is undesirable to have a large cathode surface in contact with a small anode surface. The rate of penetration from corrosion increases as the ratio of the cathode to anode surface area increases; as it decreases, the rate of penetration decreases too. This situation is portrayed using a riveted fastener as shown in Figues 1 and 2. When using a stainless steel plate with a zinc rivet (Fig. 1), the ratio of the cathode surface area to the anode surface area is large, and the rivet will fail rapidly becaaus of accelerated corrosion. When combining a zinc plate witha a stainless steel rivet (Fig. 2), the area ratio between the cathode and anode is reversed, and although more surface area is affected, the depth of penetration is small; the fastener should not fail because of corrosion. The size ocorrelation to the corrsoion rate is also shown in Table 1.
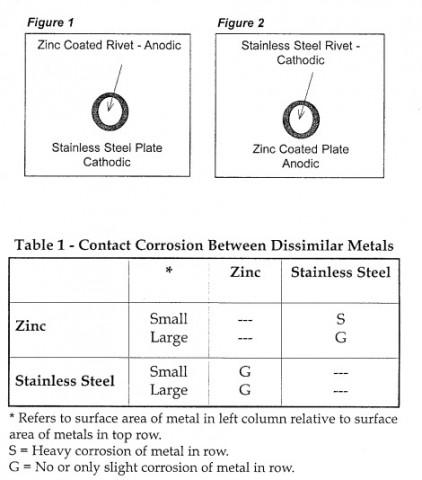
Corrosion zinc and stainless steel

